A piece of metal of mass 27 g at 93° C is placed
in a calorimeter containing 59.2 g of water a...

Chemistry, 18.10.2020 15:01 GhostElite295
A piece of metal of mass 27 g at 93° C is placed
in a calorimeter containing 59.2 g of water at
21°C. The final temperature of the mixture is 34.9 ° C. What is the specific heat capacity of the metal? Assume that there is no energy lost to the surroundings.
Answer in units of J/g. ° C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
Questions

Mathematics, 13.05.2021 01:00

Biology, 13.05.2021 01:00



Mathematics, 13.05.2021 01:00





Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00



Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00


Social Studies, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

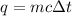 .
.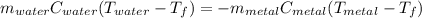
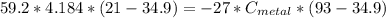
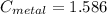 J/g°C.
J/g°C.

