
Chemistry, 18.10.2020 14:01 questions61
Explain how your observations verify that the residue in your crucible after heating is potassium chloride.
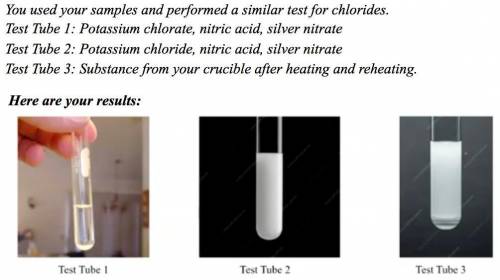

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

You know the right answer?
Explain how your observations verify that the residue in your crucible after heating is potassium ch...
Questions

History, 26.09.2019 21:50




Computers and Technology, 26.09.2019 21:50

History, 26.09.2019 21:50




Health, 26.09.2019 21:50



Biology, 26.09.2019 21:50


Biology, 26.09.2019 21:50

Mathematics, 26.09.2019 21:50

Physics, 26.09.2019 21:50

Biology, 26.09.2019 21:50

Health, 26.09.2019 21:50

Mathematics, 26.09.2019 21:50



