
Chemistry, 18.10.2020 14:01 lolagrace06
How many moles of precipitate will be formed when 100.0 mL of 0.200 M NaBr is reacted with excess Pb(NO₃)₂ in the following chemical reaction? 2 NaBr (aq) + Pb(NO₃)₂ (aq) → PbBr₂ (s) + 2 NaNO₃ (aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
How many moles of precipitate will be formed when 100.0 mL of 0.200 M NaBr is reacted with excess Pb...
Questions


Mathematics, 20.04.2021 08:40



Mathematics, 20.04.2021 08:40


English, 20.04.2021 08:40


Geography, 20.04.2021 08:40


Advanced Placement (AP), 20.04.2021 08:40

English, 20.04.2021 08:40


Mathematics, 20.04.2021 08:40

Social Studies, 20.04.2021 08:40


Mathematics, 20.04.2021 08:40



Mathematics, 20.04.2021 08:40

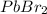 formed will be 0.01 moles.
formed will be 0.01 moles.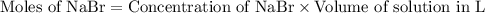
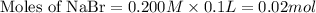
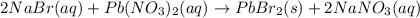
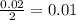 mole of
mole of 

