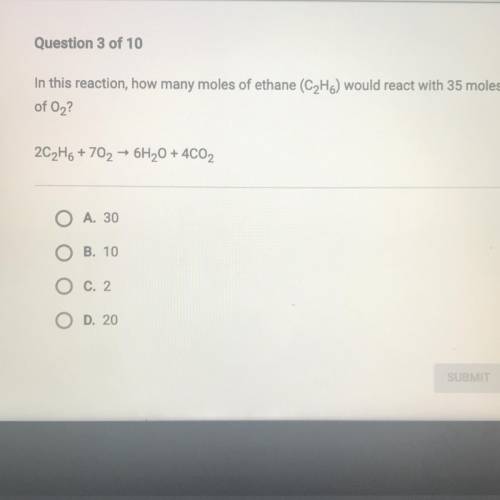
In this reaction, how many moles of ethane (C2H6) would react with 35 moles
of O2?
2C2H6 + 70...

Chemistry, 18.10.2020 04:01 tatiana4ever
In this reaction, how many moles of ethane (C2H6) would react with 35 moles
of O2?
2C2H6 + 702 + 6H20 + 4CO2
A. 30
B. 10
o
C. 2
O D. 20

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Questions





English, 07.10.2019 13:30



Health, 07.10.2019 13:30




Biology, 07.10.2019 13:30


Biology, 07.10.2019 13:30

English, 07.10.2019 13:30

Biology, 07.10.2019 13:30


Mathematics, 07.10.2019 13:30

Social Studies, 07.10.2019 13:30



