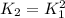Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
The equilibrium constant for A + 2B → 3C is 2.1 * 10^-6
Determine the equilibrium constant for 2A +...
Questions



Social Studies, 19.09.2019 01:30

Mathematics, 19.09.2019 01:30






Chemistry, 19.09.2019 01:30

Arts, 19.09.2019 01:30

Social Studies, 19.09.2019 01:30


Social Studies, 19.09.2019 01:30

Computers and Technology, 19.09.2019 01:30

Mathematics, 19.09.2019 01:30

Chemistry, 19.09.2019 01:30



![K_1=\frac{[C]^3}{[A][B]^2}=2.1x10^{-6}](/tpl/images/0816/9776/e7d3e.png)
![K_2=\frac{[C]^6}{[A]^2[B]^4}](/tpl/images/0816/9776/22d5b.png)