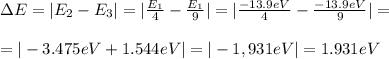Chemistry, 18.08.2019 13:00 lilyella1004
What is the energy needed to raise an electron in the hydrogen atom from the second energy level to the third energy level ?
a.) 1.52x10^4 j b.) 3.63*10^-19 j c.) 2.18x10^-19 j d.) 4.48x10^-9 j e.) 3.03*10^-19j

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
What is the energy needed to raise an electron in the hydrogen atom from the second energy level to...
Questions

Mathematics, 27.08.2020 22:01


English, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01

History, 27.08.2020 22:01

Chemistry, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01




Chemistry, 27.08.2020 22:01

Computers and Technology, 27.08.2020 22:01



World Languages, 27.08.2020 22:01

English, 27.08.2020 22:01

Biology, 27.08.2020 22:01

Social Studies, 27.08.2020 22:01

Computers and Technology, 27.08.2020 22:01