
Chemistry, 17.10.2020 08:01 payshencec21
Determine the number of moles of oxygen atoms in each of the following.
1) 4.93 mol H2O2
2) 2.01 mol N2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Determine the number of moles of oxygen atoms in each of the following.
1) 4.93 mol H2O2
2) 2...
2) 2...
Questions

Mathematics, 18.03.2021 16:20

English, 18.03.2021 16:20

Health, 18.03.2021 16:20



Chemistry, 18.03.2021 16:20




Mathematics, 18.03.2021 16:20

Mathematics, 18.03.2021 16:20

Health, 18.03.2021 16:20

Mathematics, 18.03.2021 16:20

History, 18.03.2021 16:20





History, 18.03.2021 16:20

Mathematics, 18.03.2021 16:20

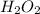 contains 9.86 moles of oxygen atoms.
contains 9.86 moles of oxygen atoms.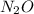 contains 2.01 moles of oxygen atoms.
contains 2.01 moles of oxygen atoms.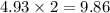 moles of oxygen atoms.
moles of oxygen atoms.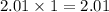 moles of oxygen atoms.
moles of oxygen atoms.

