
The ratio of the lons in an ionic compound depends on the charges of the ions. The amount of positive charge
must balance the amount of negative charge. For each compound, both the total positive charge and the total
negative charge will equal the LCM found in part B.
Determine the number of positive ions and the number of negative ions for each ionic compound in the table.
Use the lonic charges of each element you found in part A. The number of each ion times the charge on the lon
should equal the LCM determined for each cell in part B.
[# of ions of an element] * [lon charge) - least common multiple (LCM)
Drag each element's chemical symbol to the table to show how many lons are needed to balance the charges.
Each symbol will be used more than once. The first row is already completed for you.
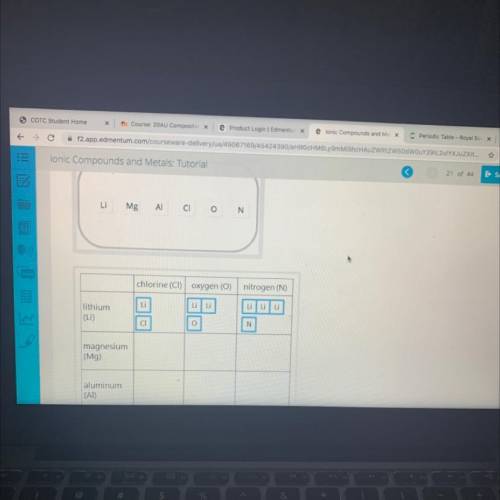

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
The ratio of the lons in an ionic compound depends on the charges of the ions. The amount of positiv...
Questions

Mathematics, 09.11.2020 16:30


Chemistry, 09.11.2020 16:30



Social Studies, 09.11.2020 16:40





Geography, 09.11.2020 16:40





Engineering, 09.11.2020 16:40

Computers and Technology, 09.11.2020 16:40






