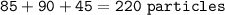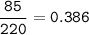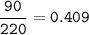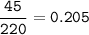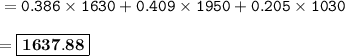Chemistry, 17.10.2020 01:01 nommies005
Calculate the average atomic mass of the mystery element given the isotopic data below.
Sample size: Isotope #1 85 - particles, Isotope #2 - 90 particles, Isotope #3 - 45 particles
Masses: Isotope #1 total mass = 1630 amu, Isotope 2 total mass = 1950 amu, Isotope #3 total mass = 1030 amu

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

You know the right answer?
Calculate the average atomic mass of the mystery element given the isotopic data below.
Sample size...
Questions

Spanish, 19.05.2021 08:30




English, 19.05.2021 08:30

Mathematics, 19.05.2021 08:30

Physics, 19.05.2021 08:30

Spanish, 19.05.2021 08:30

History, 19.05.2021 08:30

Mathematics, 19.05.2021 08:30

Mathematics, 19.05.2021 08:30

Mathematics, 19.05.2021 08:30

Mathematics, 19.05.2021 08:30

English, 19.05.2021 08:30

Chemistry, 19.05.2021 08:30

English, 19.05.2021 08:30


Chemistry, 19.05.2021 08:30

Biology, 19.05.2021 08:30