Chemistry, 16.10.2020 14:01 abelxoconda
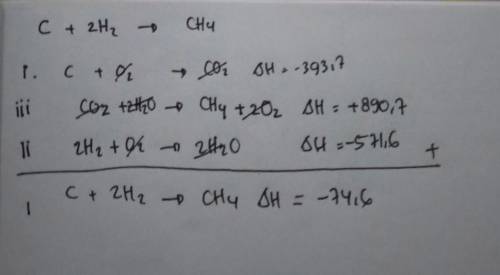
State Hess' law of constant heat summation.
(b) Calculate the enthalpy of formation of CH4 from the following data:
i) C(s) + O2(g) → CO2(g); ∆H = -393.7 kJ/mol
ii) H2(g) + 1⁄2 O2(g) → H2O(l); ∆H = -285.8 kJ/mol
iii) CH4(g) + 2 O2(g)→ CO2(g) + 2H2O(l); ∆H = -890.4 kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
State Hess' law of constant heat summation.
(b) Calculate the enthalpy of formation of CH4 from the...
Questions

English, 31.08.2019 08:10

Biology, 31.08.2019 08:10

Chemistry, 31.08.2019 08:10


History, 31.08.2019 08:10


Geography, 31.08.2019 08:10


History, 31.08.2019 08:10


Mathematics, 31.08.2019 08:10


Social Studies, 31.08.2019 08:10

Mathematics, 31.08.2019 08:10


Mathematics, 31.08.2019 08:10

Mathematics, 31.08.2019 08:10


Physics, 31.08.2019 08:10

Health, 31.08.2019 08:10