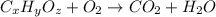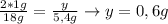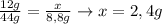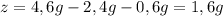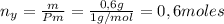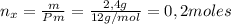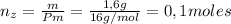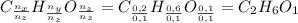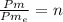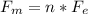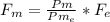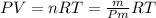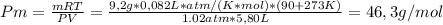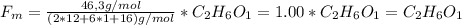Chemistry, 16.10.2020 14:01 Baby010391
Un compuesto formado por carbono, hidrógeno y oxígeno tiene una masa de 4,6 g. Se hace reaccionar con 9,6 g de oxígeno dando 8,8 g de CO2 y 5,4 g de agua. Si cogemos 9,2 g de un compuesto en un volumen 5,80l en P= 780 mmHg a una temperatura de 90ºC. Calcula la fórmula empírica y molecular.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2


Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3

Chemistry, 23.06.2019 16:10
Which quote from the passage best illustrates della’s optimism? “…and at length she was able to look up with dim eyes and a smile and say: "my hair grows so fast, jim! ” “…her heart had simply craved and yearned over them without the least hope of possession.” “and now, they were hers, but the tresses that should have adorned the coveted adornments were gone.” “beautiful combs, pure tortoise shell, with jewelled rims--just the shade to wear in the beautiful vanished hair.”d, ardent, most likely means? gloomy calm passionate innocent
Answers: 2
You know the right answer?
Un compuesto formado por carbono, hidrógeno y oxígeno tiene una masa de 4,6 g. Se hace reaccionar co...
Questions



Mathematics, 25.02.2021 19:40

Social Studies, 25.02.2021 19:40


English, 25.02.2021 19:40






Biology, 25.02.2021 19:40



Mathematics, 25.02.2021 19:40

Computers and Technology, 25.02.2021 19:40


Mathematics, 25.02.2021 19:40

Mathematics, 25.02.2021 19:40