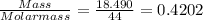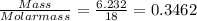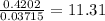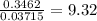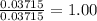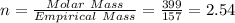Chemistry, 29.08.2019 12:10 cdvazquez727
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of the compound gave 18.490 mg of co2 and 6.232 mg of h2o. this compound was found to have a molecular mass of 399. what is the molecular formula of this compound? put your answer in form of cxhyoz.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2


Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of...
Questions

Mathematics, 28.09.2019 16:30




Mathematics, 28.09.2019 16:30

Health, 28.09.2019 16:30



Mathematics, 28.09.2019 16:30

Mathematics, 28.09.2019 16:30

Social Studies, 28.09.2019 16:30



Mathematics, 28.09.2019 16:30


Mathematics, 28.09.2019 16:30

Mathematics, 28.09.2019 16:30


Health, 28.09.2019 16:30