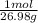Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
1. Calculate the number of moles in each of the following masses:
a. 64.1 g of aluminum ans: 2.38 m...
Questions

Mathematics, 03.04.2021 01:00

English, 03.04.2021 01:00

Biology, 03.04.2021 01:00

Mathematics, 03.04.2021 01:00





Mathematics, 03.04.2021 01:00

Physics, 03.04.2021 01:00

Mathematics, 03.04.2021 01:00



Mathematics, 03.04.2021 01:00



History, 03.04.2021 01:00


Mathematics, 03.04.2021 01:00

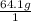 *
*