
Chemistry, 16.10.2020 20:01 hargunk329
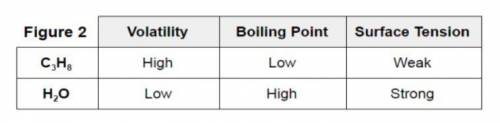
Consider the information provided in experiment #3 Figure 2. Three different variables are compared for C3H8 (propane) and H2O (water). The strength of the intermolecular forces are responsible for the behavior of the two compounds.
Identify the intermolecular force for the two compounds.
Which one is stronger?
Explain how the strength of intermolecular forces plays a role in at least two of the properties (volatility, boiling point, or surface tension) for C3H8 (propane) and H2O (water).
Cite evidence from the data to explain your reasoning.
Experiment #3: The student follows study #2 with a comparison of propane (C3H8) and water. Volatility is the tendency for a substance to vaporize and it was found that propane vaporizes quickly while water vaporized slowly. Surface tension is a physical property equal to the amount of force per unit area necessary to expand the surface of a liquid. It is this phenomenon which allows a water strider or lizard to run across the surface of water.
100 points

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
Consider the information provided in experiment #3 Figure 2. Three different variables are compared...
Questions

Mathematics, 14.01.2020 20:31

History, 14.01.2020 20:31

Computers and Technology, 14.01.2020 20:31

History, 14.01.2020 20:31

Business, 14.01.2020 20:31








Mathematics, 14.01.2020 20:31



Mathematics, 14.01.2020 20:31



Mathematics, 14.01.2020 20:31



