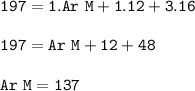Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
The relative formula mass (M) of a Group 2 metal carbonate is 197
Relative atomic masses (Ar): C =...
Questions



Mathematics, 10.11.2020 03:10







Mathematics, 10.11.2020 03:10

Mathematics, 10.11.2020 03:10


History, 10.11.2020 03:10

Mathematics, 10.11.2020 03:10

Biology, 10.11.2020 03:10

Mathematics, 10.11.2020 03:10

Mathematics, 10.11.2020 03:10

English, 10.11.2020 03:10

Mathematics, 10.11.2020 03:10

Mathematics, 10.11.2020 03:10