

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1

Chemistry, 23.06.2019 14:20
Timed ! in which of these statements are protons, electrons, and neutrons correctly compared? quarks are present in protons and neutrons but not in electrons. quarks are present in protons, neutrons, and electrons. quarks are present in neutrons and electrons but not in protons. quarks are present in protons and electrons but not in neutrons.
Answers: 1

Chemistry, 23.06.2019 15:00
Does the formation of all chemical bonds is based on sharing of electrons?
Answers: 1
You know the right answer?
How many molecules of sucrose (c12h22o11, molar mass = 342.30 g/mol) are contained in
14.3 ml...
14.3 ml...
Questions

English, 01.04.2021 20:50


Mathematics, 01.04.2021 20:50

Arts, 01.04.2021 20:50

English, 01.04.2021 20:50


Mathematics, 01.04.2021 20:50

Mathematics, 01.04.2021 20:50





History, 01.04.2021 20:50


Mathematics, 01.04.2021 20:50



Mathematics, 01.04.2021 20:50

Mathematics, 01.04.2021 20:50

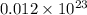 molecules
molecules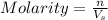
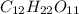 = ?
= ?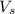 = volume of solution in liter = 0.0143L
= volume of solution in liter = 0.0143L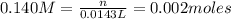
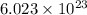 molecules of sucrose.
molecules of sucrose.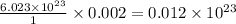 molecules of sucrose.
molecules of sucrose.

