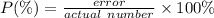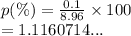Chemistry, 15.10.2020 05:01 saskiat8549
The accepted density of copper is 8.96 g/mL. Calculate the percent error of a student’s measurement which resulted in a density of 8.86 g/mL.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1


Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
The accepted density of copper is 8.96 g/mL. Calculate the percent error of a student’s measurement...
Questions


Mathematics, 10.04.2020 17:33



Social Studies, 10.04.2020 17:33





Chemistry, 10.04.2020 17:34

English, 10.04.2020 17:34






Computers and Technology, 10.04.2020 17:34

Mathematics, 10.04.2020 17:34