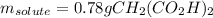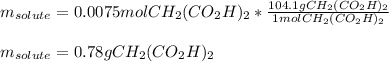Chemistry, 15.10.2020 02:01 marelinatalia2000
50.00 mL of a solution containing 0.15 M CH2 (CO2 H)2 and 0.020 M MnSO4
1. Calculate the mass in g of malonic acid required.
Watch your sig figs! (enter just the number)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2


Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1
You know the right answer?
50.00 mL of a solution containing 0.15 M CH2 (CO2 H)2 and 0.020 M MnSO4
1. Calculate the mass in g...
Questions

Mathematics, 06.12.2019 03:31


History, 06.12.2019 03:31



Biology, 06.12.2019 03:31








English, 06.12.2019 03:31


English, 06.12.2019 03:31



Chemistry, 06.12.2019 03:31