Kc for the reaction of hydrogen and iodine to produce hydrogen iodide.
H2(g) + I2(g) ⇌ 2HI(g)
...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Questions




Social Studies, 07.01.2020 04:31

Mathematics, 07.01.2020 04:31

History, 07.01.2020 04:31


History, 07.01.2020 04:31


Mathematics, 07.01.2020 04:31


History, 07.01.2020 04:31


Chemistry, 07.01.2020 04:31

History, 07.01.2020 04:31



Mathematics, 07.01.2020 04:31

Social Studies, 07.01.2020 04:31

Mathematics, 07.01.2020 04:31

![[H_2]_{eq}=0.183M](/tpl/images/0806/0147/ac24e.png)
![[I_2]_{eq}=0.183M](/tpl/images/0806/0147/bd3cc.png)
![[HI]_{eq}=0.025M](/tpl/images/0806/0147/6a579.png)
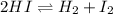
![Kc=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0806/0147/d1f0c.png)
 turns out:
turns out:![Kc=\frac{x*x}{([HI]_0-2x)^2}\\\\54.3=\frac{x^2}{(0.391M-2x)^2}](/tpl/images/0806/0147/70f4c.png)
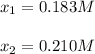
![[HI]_{eq}=0.391M-2*0.183M=0.025M](/tpl/images/0806/0147/7d0d9.png)


