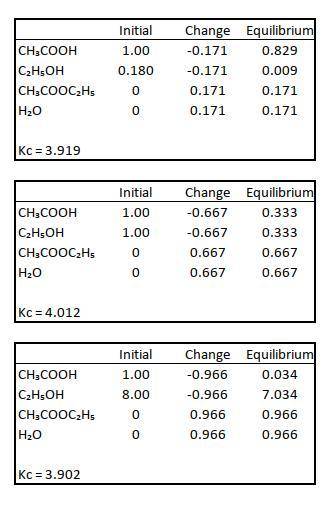
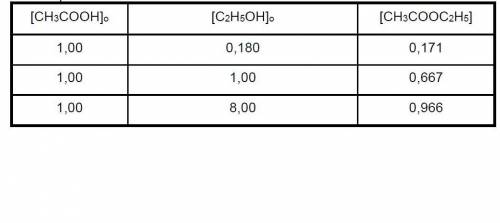
Data for CH3COOH(l) + C2H5OH(l) CH3COOC2H5(l) + H2O(l) balance were obtained at 100. The initial concentrations of the reagents are indicated in columns 1 and 2 of the table below and the CH3COOC2H5 concentrations in equilibrium are given in column 3. Calculate H2O and determine the value of KC

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Data for CH3COOH(l) + C2H5OH(l) CH3COOC2H5(l) + H2O(l) balance were obtained at 100. The initial con...
Questions

Mathematics, 09.06.2021 20:10

Physics, 09.06.2021 20:10



Mathematics, 09.06.2021 20:10


English, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10


Mathematics, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10



Mathematics, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10

Mathematics, 09.06.2021 20:10