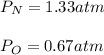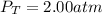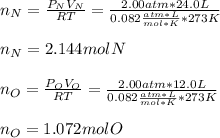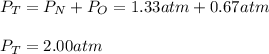In a chemistry laboratory, a student filled a 10.0 L container with two (2) different gases. The
gases are nitrogen gas taken from 24.0 L container at 2.00 atm and 12.0 L container of
oxygen at 2.00 atm. If the temperature of the gases is 273 K, calculate the partial pressure
of both gases in the resulting mixture and the total pressure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
In a chemistry laboratory, a student filled a 10.0 L container with two (2) different gases. The
ga...
Questions



Mathematics, 08.06.2021 05:20




Chemistry, 08.06.2021 05:20

History, 08.06.2021 05:20


Mathematics, 08.06.2021 05:20

Mathematics, 08.06.2021 05:20


History, 08.06.2021 05:20

Mathematics, 08.06.2021 05:20





Mathematics, 08.06.2021 05:20