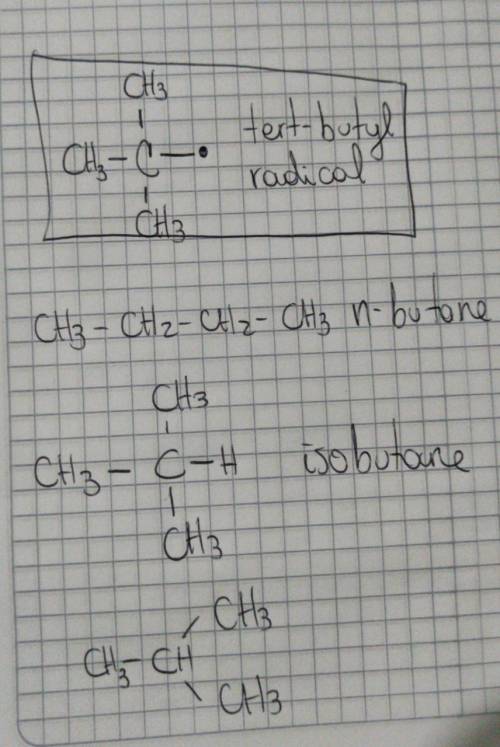
The molecular formula of butane is C4H10. It is obtained from petroleum and is used commonly in LPG (Liquefied Petroleum Gas) cylinders (a common source of cooking gas). It has two arrangements of carbon atoms: a straight chain and a branched chain. Using this information, draw the structure of the tertiary butyl radical that will form upon removal of a hydrogen atom. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
The molecular formula of butane is C4H10. It is obtained from petroleum and is used commonly in LPG...
Questions


English, 01.07.2020 23:01

Chemistry, 01.07.2020 23:01

Mathematics, 01.07.2020 23:01

Mathematics, 01.07.2020 23:01

Mathematics, 01.07.2020 23:01




Social Studies, 01.07.2020 23:01


Mathematics, 01.07.2020 23:01

Mathematics, 01.07.2020 23:01

Mathematics, 01.07.2020 23:01


Computers and Technology, 01.07.2020 23:01


English, 01.07.2020 23:01

Mathematics, 01.07.2020 23:01

Mathematics, 01.07.2020 23:01