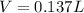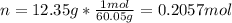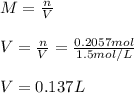Chemistry, 13.10.2020 01:01 onlymyworld27
It takes 12.35 grams of acetic acid (CH3COOH; molar mass of 60.05 g/mol) to prepare a 1.5 M solution. What is the volume in L?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

You know the right answer?
It takes 12.35 grams of acetic acid (CH3COOH; molar mass of 60.05 g/mol) to prepare a
1.5 M solutio...
Questions


History, 10.03.2021 14:50


English, 10.03.2021 14:50

Mathematics, 10.03.2021 14:50

Mathematics, 10.03.2021 14:50



Mathematics, 10.03.2021 14:50

Mathematics, 10.03.2021 14:50

English, 10.03.2021 14:50


Business, 10.03.2021 14:50

Mathematics, 10.03.2021 14:50

English, 10.03.2021 14:50


Biology, 10.03.2021 14:50

Mathematics, 10.03.2021 14:50