
Chemistry, 12.10.2020 20:01 LuluMathLover101
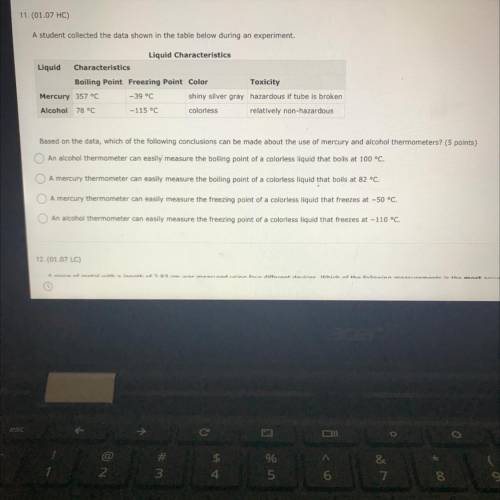
A student collected the data shown in the table below during an experiment.
Liquid Characteristics
Liquid Characteristics
Boiling Point Freezing Point Color
Toxicity
Mercury 357 °C -39 °C
shiny silver gray hazardous if tube is broken
Alcohol 78 °C
- 115 °C colorless relatively non-hazardous
Based on the data, which of the following conclusions can be made about the use of mercury and alcohol thermometers? (5 points)
An alcohol thermometer can easily measure the boiling point of a colorless liquid that boils at 100 °C.
A mercury thermometer can easily measure the boiling point of a colorless liquid that boils at 82 °C.
A mercury thermometer can easily measure the freezing point of a colorless liquid that freezes at -50 °C.
An alcohol thermometer can easily measure the freezing point of a colorless liquid that freezes at -110 °C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
A student collected the data shown in the table below during an experiment.
Liquid Characteristics<...
Questions


Advanced Placement (AP), 02.07.2019 16:30




Mathematics, 02.07.2019 16:30

Mathematics, 02.07.2019 16:30

Mathematics, 02.07.2019 16:30


Mathematics, 02.07.2019 16:30





Biology, 02.07.2019 16:30


Mathematics, 02.07.2019 16:30

Geography, 02.07.2019 16:30


History, 02.07.2019 16:30



