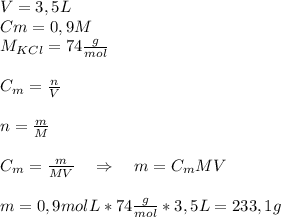How would you prepare 3.5 l of a 0.9m solution of kcl?
a. add 23 g of kcl to a 3.5 l contain...

How would you prepare 3.5 l of a 0.9m solution of kcl?
a. add 23 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.
b. add 233 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.
c. add 567 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.
d. add 287 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1
You know the right answer?
Questions



Mathematics, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00



Mathematics, 09.12.2020 05:00

Chemistry, 09.12.2020 05:00




Spanish, 09.12.2020 05:00


Mathematics, 09.12.2020 05:00



Computers and Technology, 09.12.2020 05:00

Mathematics, 09.12.2020 05:00

English, 09.12.2020 05:00

Chemistry, 09.12.2020 05:00