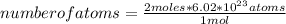Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
The molar mass of magnesium (mg) is 24.30 g/mol. there are 6.02 1023 atoms in 1 mol.
ho...
ho...
Questions


Mathematics, 31.08.2021 16:10


Mathematics, 31.08.2021 16:10




History, 31.08.2021 16:10


Mathematics, 31.08.2021 16:10

Mathematics, 31.08.2021 16:10

Biology, 31.08.2021 16:10

English, 31.08.2021 16:10


Computers and Technology, 31.08.2021 16:10

Social Studies, 31.08.2021 16:10



Social Studies, 31.08.2021 16:10

English, 31.08.2021 16:10


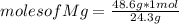
 atoms. And you know that 48.6 grams are 2 moles. Then, you can reapply a simple rule of three: if in one mole there are
atoms. And you know that 48.6 grams are 2 moles. Then, you can reapply a simple rule of three: if in one mole there are