
Chemistry, 11.10.2020 14:01 ohernandez35
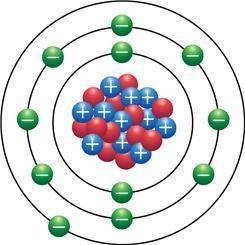
In the Bohr model of an atom (shown), protons are represented in blue, the neutrons are red and the electrons are green. How reactive is the element that this model represents?
the answer choices :
a. Highly reactive because the model has two complete electron shells
b. Highly reactive because the model is showing only one valence electron.
c. Non-reactive because the model is showing only one valence electron
d. Non-reactive because the model is showing two complete electron shells

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
In the Bohr model of an atom (shown), protons are represented in blue, the neutrons are red and the...
Questions



Biology, 30.10.2019 03:31

Social Studies, 30.10.2019 03:31


Mathematics, 30.10.2019 03:31


English, 30.10.2019 03:31



Computers and Technology, 30.10.2019 03:31

Health, 30.10.2019 03:31

Mathematics, 30.10.2019 03:31

Mathematics, 30.10.2019 03:31








