Chemistry, 11.10.2020 01:01 officialrogerfp3gf2s
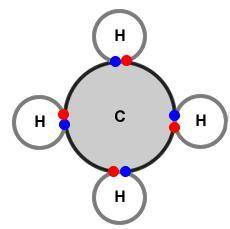
What conclusion about bonding can be drawn from these diagrams?
A) In ionic bonding the valence electrons are combined while the valence electrons are electrostatically portioned during non-polar covalent bonding.
B) The valence electrons are shared between both atoms in covalent bonding while the valence electrons are completely transferred in ionic bonding.
C) Valence electrons reside in a sea of electrons in metallic bonding while valence electrons are permanently exchanged during crystalline bonding.
D) During amorphous bonding the valence electrons are unevenly shared between the atoms while the valence electrons are borrowed in polar covalent bonding.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
What conclusion about bonding can be drawn from these diagrams?
A) In ionic bonding the valence ele...
Questions

Mathematics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00


Physics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00




Mathematics, 06.03.2021 01:00


Biology, 06.03.2021 01:00




Mathematics, 06.03.2021 01:00


Mathematics, 06.03.2021 01:00



English, 06.03.2021 01:00