
Chemistry, 10.10.2020 22:01 mrashrafkotkaat
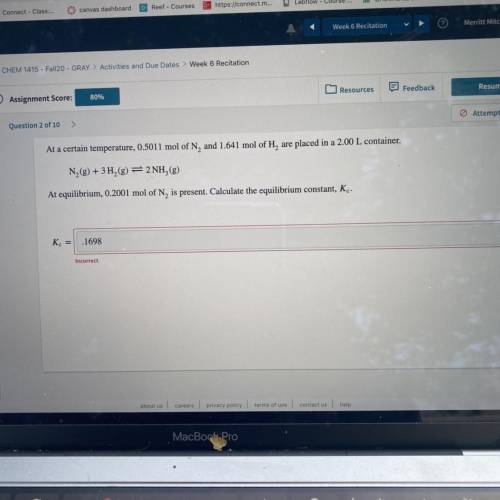
At a certain temperature, 0.5011 mol of N, and 1.641 mol of H, are placed in a 2.00 L container.
N2(g) + 3H2(g) = 2NH,(g)
At equilibrium, 0.2001 mol of N, is present. Calculate the equilibrium constant, Kc.
K =
.1698
Incorrect
about us
careers
privacy policy
terms of use
contact Us
help

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
At a certain temperature, 0.5011 mol of N, and 1.641 mol of H, are placed in a 2.00 L container.
N2...
Questions


Mathematics, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20


Mathematics, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20


History, 12.03.2021 01:20

Mathematics, 12.03.2021 01:20


English, 12.03.2021 01:20








Mathematics, 12.03.2021 01:20



