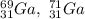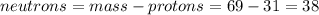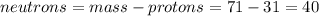Chemistry, 08.10.2020 14:01 norahfrost
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu.
Part A. How many protons and neutrons are in the nucleus of isotope with mass of 68.926 amu? Express your answers as an integers. Enter your answers numerically separated by a comma. p, n = .
Part B. How many protons and neutrons are in the nucleus of isotope with mass of 70.925 amu? Express your answers as an integers. Enter your answers numerically separated by a comma. p, n = .
Part C. Write the complete atomic symbol for each, showing the atomic number and mass number. Express your answers as isotopes separated by a comma.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu.
Par...
Questions






Mathematics, 18.02.2020 00:02





Mathematics, 18.02.2020 00:02

Social Studies, 18.02.2020 00:02







Mathematics, 18.02.2020 00:02