Chemistry, 08.10.2020 14:01 justijust500
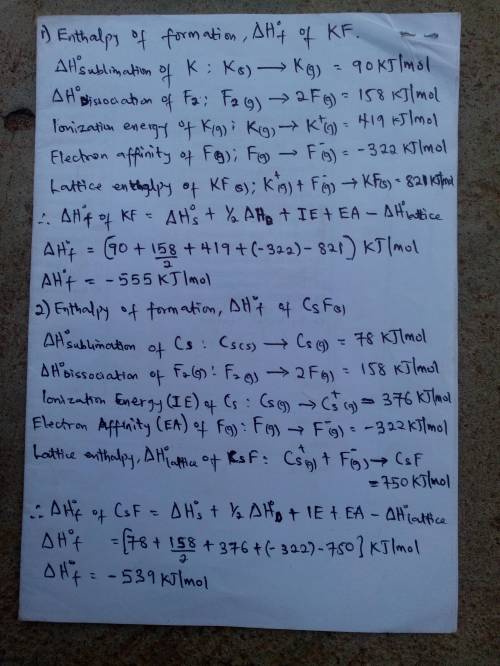
Calculate the enthalpies of formation, ΔHf∘, of the group 1 fluoride compounds from their elements using the Born–Haber cycle.
Process H∘, /
sublimation of K(s) 90
sublimation of Cs(s) 78
dissociation of F2(g) 158
ionization energy of K(g) 419
ionization energy of Cs(g) 376
electron affinity of F(g) −322
lattice enthalpy of KF(s) 821
lattice enthalpy of CsF(s) 750

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
Calculate the enthalpies of formation, ΔHf∘, of the group 1 fluoride compounds from their elements u...
Questions

Social Studies, 24.07.2019 14:40

History, 24.07.2019 14:40

History, 24.07.2019 14:40



Chemistry, 24.07.2019 14:40




History, 24.07.2019 14:40

Biology, 24.07.2019 14:40

History, 24.07.2019 14:40

Mathematics, 24.07.2019 14:40





Social Studies, 24.07.2019 14:50

Biology, 24.07.2019 14:50

Mathematics, 24.07.2019 14:50