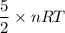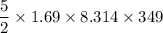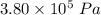A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.70 ✕ 105 Pa. The temperature of the air outside the tank is 19.0°C. The molar mass of Cl2 is 70.9 g/mol. (a) What is the volume of the tank (in m3)? m3 (b) What is the internal energy of the gas (in J)? J (c) What is the work done by the gas (in J) if the temperature and pressure inside the tank drop to 31.0°C and 3.80 ✕ 105 Pa, respectively, due to a leak? (Assume that the air outside the tank can be treated as a vacuum.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.7...
Questions


Chemistry, 30.09.2021 22:50

Mathematics, 30.09.2021 22:50

Mathematics, 30.09.2021 22:50



English, 30.09.2021 22:50


Mathematics, 30.09.2021 22:50


Biology, 30.09.2021 22:50


Social Studies, 30.09.2021 22:50



Advanced Placement (AP), 30.09.2021 22:50

Biology, 30.09.2021 22:50

English, 30.09.2021 22:50


English, 30.09.2021 22:50

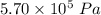
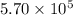
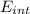 =
=