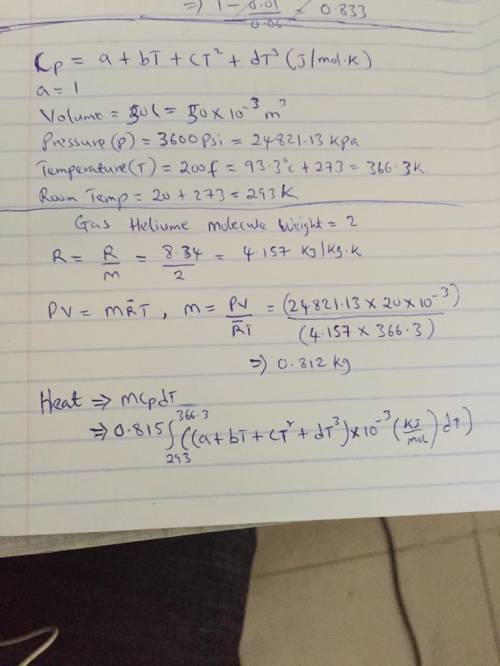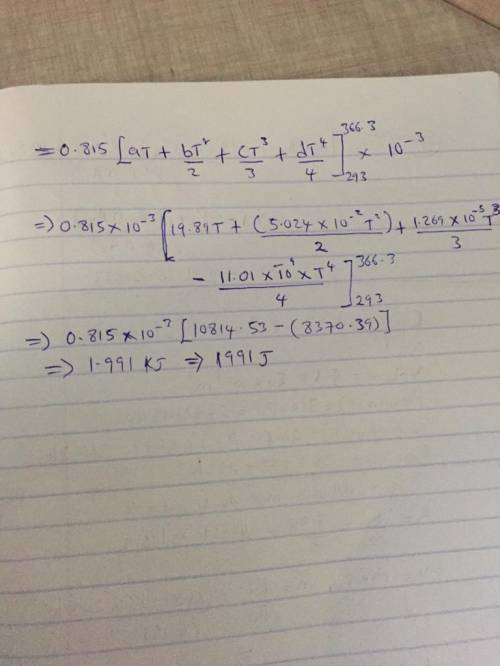The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial: �;<<< = � + �� + ��C + ��E, with units J/(mol K). For methane (CH4) the coefficients are � = 19.89, � = 5.024 × 10NC, � = 1.269 × 10NO, � = −11.01 × 10NQ. At a production facility, the gas in a 50-liter tank is compressed to 3,600 psi (gage) during which time the temperature rises to 200°F. How much heat in J is given off as the gas cools to room temperature? Suppose the compressed gas is helium, how much heat would be given off in this case?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial...
Questions



Mathematics, 23.01.2020 18:31


Mathematics, 23.01.2020 18:31




Mathematics, 23.01.2020 18:31


Biology, 23.01.2020 18:31



Biology, 23.01.2020 18:31

History, 23.01.2020 18:31




Business, 23.01.2020 18:31

English, 23.01.2020 18:31