
Chemistry, 08.10.2020 01:01 Jasminehenry123
answer the questions below, using lt (for is less than), gt (for is greater than), eq (for equal to), or mi (for more information) in the blanks provided. 1. the wavelength of the photon required to promote an electron in the hydrogen atom from the n = 1 to the n = 3 level is the wavelength of the photon required to promote an electron in the hydrogen atom from the n =1 to the n = 2 level. 2. the energy of a photon with a wavelength of 463 nm is the energy of a photon whose wavelength is 722 nm. 3. in order to promote an electron to go to a higher energy level, light with a wavelength that is 400 nm is required.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
What is the molar mass of babr2? a. 217.2 g/mol b. 297.1 g/mol c. 354.5 g/mol d. 434.4 g/mol
Answers: 1

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

You know the right answer?
answer the questions below, using lt (for is less than), gt (for is greater than), eq (for equal to)...
Questions

Mathematics, 03.07.2019 13:00




Social Studies, 03.07.2019 13:00

Social Studies, 03.07.2019 13:00

English, 03.07.2019 13:00

English, 03.07.2019 13:00


Mathematics, 03.07.2019 13:00

Mathematics, 03.07.2019 13:00




Mathematics, 03.07.2019 13:00


Mathematics, 03.07.2019 13:00

History, 03.07.2019 13:00


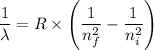
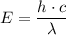
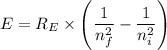
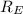 = -2.178 × 10⁻¹⁸ J
= -2.178 × 10⁻¹⁸ J 

