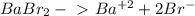Chemistry, 15.10.2019 04:30 DEVORIA2001
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–4 g babr2 in 1.00 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3


Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–...
Questions

Social Studies, 19.04.2021 05:10


Chemistry, 19.04.2021 05:10


Mathematics, 19.04.2021 05:10



Mathematics, 19.04.2021 05:10

Social Studies, 19.04.2021 05:10


Chemistry, 19.04.2021 05:10

Mathematics, 19.04.2021 05:10



Biology, 19.04.2021 05:10