Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
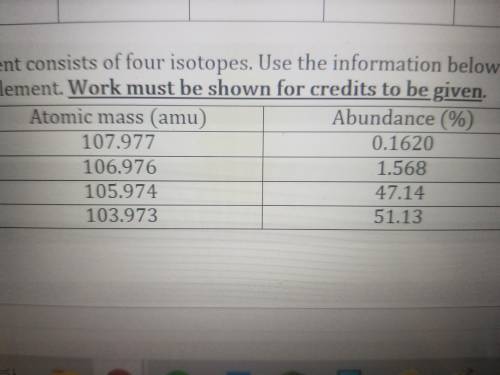
A hypothetical element consists of four isotopes. Use the information below to calculate the average...
Questions

English, 24.02.2021 18:10

Mathematics, 24.02.2021 18:10

History, 24.02.2021 18:10




Business, 24.02.2021 18:10




History, 24.02.2021 18:10





Mathematics, 24.02.2021 18:10

History, 24.02.2021 18:10

Mathematics, 24.02.2021 18:10