
Chemistry, 06.10.2020 14:01 amcdonald009
6.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 46. g/mol, is burned completely in excess oxygen and the mass of the products carefully measured:
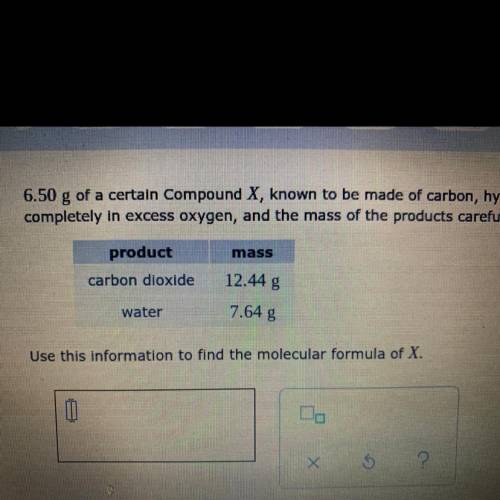

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
6.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have...
Questions

Chemistry, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30



English, 12.12.2020 16:30

History, 12.12.2020 16:30


English, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30


Biology, 12.12.2020 16:30

Computers and Technology, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30



Mathematics, 12.12.2020 16:30

Social Studies, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30



