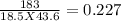Chemistry, 12.10.2019 07:30 sadiyah1116
Calculate the specific heat (j/g∘c) for a 18.5-g sample of tin that absorbs 183 j when temperature increases from 35.0 ∘c to 78.6 ∘c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Calculate the specific heat (j/g∘c) for a 18.5-g sample of tin that absorbs 183 j when temperature i...
Questions





Computers and Technology, 09.02.2021 01:20

Mathematics, 09.02.2021 01:20


Mathematics, 09.02.2021 01:20


Computers and Technology, 09.02.2021 01:20






English, 09.02.2021 01:20

Mathematics, 09.02.2021 01:20

Mathematics, 09.02.2021 01:20

Spanish, 09.02.2021 01:20