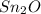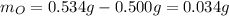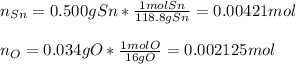Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
A 0.500 g sample of tin (Sn) is reacted with oxygen to give 0.534 g of product. What is the empirica...
Questions

Mathematics, 05.10.2020 14:01





Mathematics, 05.10.2020 14:01





Biology, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01


Chemistry, 05.10.2020 14:01


Chemistry, 05.10.2020 14:01


English, 05.10.2020 14:01