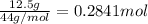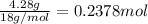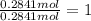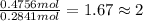Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3


Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Complete combustion of 3.90 g of a hydrocarbon produced 12.5 g of co2 and 4.28 g of h2o. what is the...
Questions






Mathematics, 05.05.2020 02:31


English, 05.05.2020 02:31


Mathematics, 05.05.2020 02:31

Mathematics, 05.05.2020 02:31

Mathematics, 05.05.2020 02:32

Mathematics, 05.05.2020 02:32

History, 05.05.2020 02:32






English, 05.05.2020 02:32

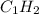 .
.