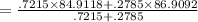Chemistry, 04.10.2020 14:01 Andinojose
Rubidium has two naturally occurring isotopes: 85Rb, with mass 84.9118 amu and natural abundance 72.15%; and 87Rb, with mass 86.9092 amu and natural abundance 27.85%. Calculate the atomic mass of rubidium. View Available Hint(s) Rubidium has two naturally occurring isotopes: 85Rb, with mass 84.9118 amu and natural abundance 72.15%; and 87Rb, with mass 86.9092 amu and natural abundance 27.85%. Calculate the atomic mass of rubidium. 86.3529 amu 84.9118 amu 85.9105 amu 85.4681 amu

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1


Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Rubidium has two naturally occurring isotopes: 85Rb, with mass 84.9118 amu and natural abundance 72....
Questions

History, 17.11.2020 22:40




Law, 17.11.2020 22:40


Mathematics, 17.11.2020 22:40


Arts, 17.11.2020 22:40

SAT, 17.11.2020 22:40

English, 17.11.2020 22:40

English, 17.11.2020 22:40



Mathematics, 17.11.2020 22:40





Mathematics, 17.11.2020 22:40