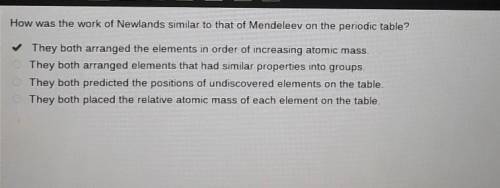
How was the work of Newlands similar to that of Mendeleev on the periodic table?
They both arranged the elements in order of increasing atomic mass.
They both arranged elements that had similar properties into groups.
They both predicted the positions of undiscovered elements on the table.
They both placed the relative atomic mass of each element on the table.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

You know the right answer?
How was the work of Newlands similar to that of Mendeleev on the periodic table?
They both arranged...
Questions




Computers and Technology, 20.08.2019 15:30


Physics, 20.08.2019 15:30



Mathematics, 20.08.2019 15:30




Mathematics, 20.08.2019 15:30

History, 20.08.2019 15:30






English, 20.08.2019 15:50