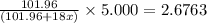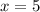Chemistry, 25.09.2020 06:01 witchhunt666
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully in a vacuum oven until no more mass was lost from the sample. After heating, the final weight of the material was 2.6763 g. What was the formula of the hydrated alumina, Al2O3•xH2O? (Enter a whole number for "x") (mol. wt. Al2O3 = 101.96)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully...
Questions




Mathematics, 12.02.2021 05:20






Mathematics, 12.02.2021 05:20


Mathematics, 12.02.2021 05:20

Mathematics, 12.02.2021 05:20

Mathematics, 12.02.2021 05:20


Mathematics, 12.02.2021 05:20




Mathematics, 12.02.2021 05:20

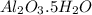
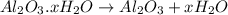
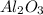 = 101.96 g/mol
= 101.96 g/mol
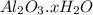 decomposes to give 101.96 g of
decomposes to give 101.96 g of 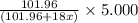 of H_2O
of H_2O