Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
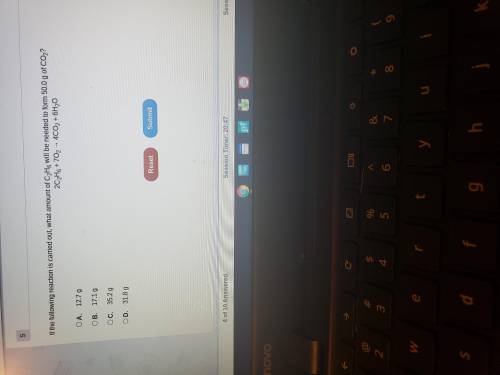
If the following reaction is carried out what amount of C2H6 will be needed to form 50.0 g of CO2
Questions


Computers and Technology, 29.08.2019 17:10

Computers and Technology, 29.08.2019 17:10

Mathematics, 29.08.2019 17:10


Computers and Technology, 29.08.2019 17:10

Computers and Technology, 29.08.2019 17:10



Chemistry, 29.08.2019 17:10


Computers and Technology, 29.08.2019 17:10


Geography, 29.08.2019 17:10