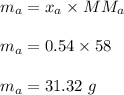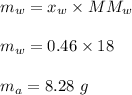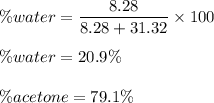Chemistry, 24.09.2020 21:01 schoolboyq3017
A mixture of water and acetone at 756 mm boils at 70.0°C. The vapor pressure of acetone is
1.54 atm at 70.0°C, while the vapor pressure of water is 0.312 atm at the same temperature.
Calculate the percentage composition of the mixture

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
A mixture of water and acetone at 756 mm boils at 70.0°C. The vapor pressure of acetone is
1.54 atm...
Questions

Mathematics, 30.07.2019 21:30

English, 30.07.2019 21:30


Chemistry, 30.07.2019 21:30

Mathematics, 30.07.2019 21:30




Mathematics, 30.07.2019 21:30







Social Studies, 30.07.2019 21:30


Mathematics, 30.07.2019 21:30


 ......( 1 )
......( 1 )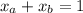 ......( 2 )
......( 2 )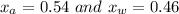 .
.