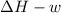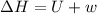Chemistry, 24.09.2020 21:01 MalikaJones
One mole of liquid water at 100°C is heated until the liquid is converted entirely to vapor at 100°C and 1 bar pressure. Calculate q, w, U, and H for each of the following:
A. The vaporization is carried out in a cylinder where the external pressure on the piston is maintained at 1 bar throughout.
B. The cylinder is first expanded against a vacuum (Pex=0) to the same volumn as in part (a), and then sufficient heat is added to vaporize the liquid completely to 1 atm pressure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2


Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
One mole of liquid water at 100°C is heated until the liquid is converted entirely to vapor at 100°C...
Questions


Advanced Placement (AP), 12.05.2021 21:40


Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40




Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

Health, 12.05.2021 21:40


Mathematics, 12.05.2021 21:40


Mathematics, 12.05.2021 21:40

Mathematics, 12.05.2021 21:40

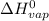 = 2260 kJ/kg
= 2260 kJ/kg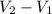 )
)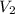 = specific volume of steam = 0.0305 m³/mol
= specific volume of steam = 0.0305 m³/mol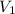 = specific volume of water = 0.00001837 m³/mol
= specific volume of water = 0.00001837 m³/mol 3.05 kJ
3.05 kJ