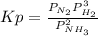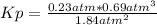Chemistry, 24.09.2020 07:01 20jessicacabriales
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a flask with of ammonia gas, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be . Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions


History, 05.05.2020 05:22



Health, 05.05.2020 05:22

History, 05.05.2020 05:22

Mathematics, 05.05.2020 05:22

History, 05.05.2020 05:22





Mathematics, 05.05.2020 05:22



Business, 05.05.2020 05:22



Mathematics, 05.05.2020 05:22

Mathematics, 05.05.2020 05:22