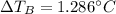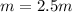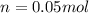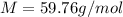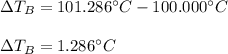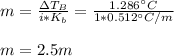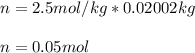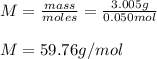Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling point increases from 100.000 OC to 101.286 OC. Determine the TB, molality, moles, and molecular weight for the solute if kb for water is 0.512 OC/m. Report each value using the correct number of significant digits. Refer to Example 1.2 and pages 3-4 in the chapter 1 notes for general chemistry 1 to understand significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling...
Questions

Mathematics, 12.01.2020 10:31

History, 12.01.2020 10:31







Biology, 12.01.2020 10:31




Mathematics, 12.01.2020 10:31

Mathematics, 12.01.2020 10:31

Mathematics, 12.01.2020 10:31

Biology, 12.01.2020 10:31


Social Studies, 12.01.2020 10:31

History, 12.01.2020 10:31

History, 12.01.2020 10:31