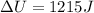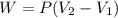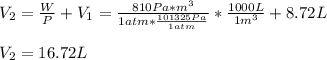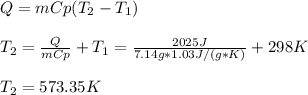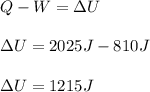Chemistry, 23.09.2020 18:01 natalie2sheffield
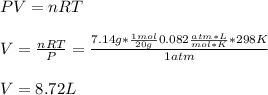
A system is composed of 7.14 grams of Ne gas at 298 K and 1 atm. When 2025 joules of heat are added to the system at constant pressure, the resultant expansion causes the system to perform 810 joules of work. Calculate the following. (a) The initial state variables (P, V, T) (b) The final state variables. (c) The change in internal energy for the process. The molecular weight of Ne is 20 and you can assume Ne behaves as an ideal gas. (

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2


Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
A system is composed of 7.14 grams of Ne gas at 298 K and 1 atm. When 2025 joules of heat are added...
Questions


History, 16.11.2019 08:31

Advanced Placement (AP), 16.11.2019 08:31



Mathematics, 16.11.2019 08:31


History, 16.11.2019 08:31

Mathematics, 16.11.2019 08:31

History, 16.11.2019 08:31

Social Studies, 16.11.2019 08:31

Mathematics, 16.11.2019 08:31


Chemistry, 16.11.2019 08:31


History, 16.11.2019 08:31

Mathematics, 16.11.2019 08:31



Social Studies, 16.11.2019 08:31