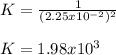Chemistry, 23.09.2020 14:01 tigistamare03
For this heterogeneous system
2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g)
the concentrations and pressures at equilibrium are [A]=2.25×10−2 M[A]=2.25×10−2 M,
PB=1.09×103 PaPB=1.09×103 Pa.
[C]=13.08 M[C]=13.08 M.
[D]=17.00 M[D]=17.00 M.
PE=3.20×104 torrPE=3.20×104 torr. Calculate the thermodynamic equilibrium constant, K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
For this heterogeneous system
2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g)
Questions


Mathematics, 12.03.2021 18:40

English, 12.03.2021 18:40

Mathematics, 12.03.2021 18:40




History, 12.03.2021 18:40

Computers and Technology, 12.03.2021 18:40

Chemistry, 12.03.2021 18:40

Mathematics, 12.03.2021 18:40



Mathematics, 12.03.2021 18:40

Advanced Placement (AP), 12.03.2021 18:40

Biology, 12.03.2021 18:40


Health, 12.03.2021 18:40


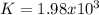
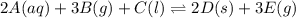
![K=\frac{1}{[A]^2}](/tpl/images/0777/3986/90aef.png)